Submissions
Submission Preparation Checklist
As part of the submission process, authors are required to check off their submission's compliance with all of the following items, and submissions may be returned to authors that do not adhere to these guidelines.- The submission has not been previously published, nor is it before another journal for consideration (or an explanation has been provided in Comments to the Editor).
- The submission file is in OpenOffice, Microsoft Word, RTF, or WordPerfect document file format.
- Where available, URLs for the references have been provided.
- The text is single-spaced; uses a 12-point font; employs italics, rather than underlining (except with URL addresses); and all illustrations, figures, and tables are placed within the text at the appropriate points, rather than at the end.
- The text adheres to the stylistic and bibliographic requirements outlined in the Author Guidelines, which is found in About the Journal.
- If submitting to a peer-reviewed section of the journal, the instructions in Ensuring a Blind Review have been followed.
Author Guidelines
Submission
As part of the submission process, the authors must verify that their application meets all the elements listed below, since otherwise the manuscripts will be rejected:
The article submitted must not have been previously published or submitted to another journal.
The document submitted must be in Open Office or Microsoft Word format.
The text must be submitted with the following characteristics: sheet size: letter, margins: 2.5x2.5x2.5x2.5 cm, font: Arial 12 points.
All illustrations, figures and tables must be in a format editable within the text immediately after they are named for the first time and not at the end of the text or as an attached file.
The text must conform to the citation guideline of the Vancouver standards for biomedical texts.
If the manuscript must go through peer review, it is necessary that the anonymity of the authors and their institution be guaranteed in the text.
Guidelines for authors
The manuscripts must be postulated in the page of the Rev Col Med Fis Rehab (www.revistacmfr.org) through the OJS platform.
When submitting the article, the author(s) must make it clear that the manuscript is an original work and has not been published by any means, nor is it being evaluated by any other printed or electronic publication. This must be done by means of a letter that must be signed by all the authors.
Form and preparation
The manuscript must be sent with a first page of identification containing the title of the article and the names of the authors in the order in which they have decided to appear in the publication. The data of the authors (names and surnames) must be complete (without initials) according to how they want to appear in the publication; regarding the affiliation, for each author they must send the highest degree of academic training and the institutional affiliation indicating the name of the institution, the section to which they belong, the city, the country and their ORCID. Following this page, each of the authors must send a signed declaration of conflict of interest.
Declaration of interest format
A corresponding author must be selected and the following information must be included: Name, institutional affiliation, email address, city, country and contact telephone number.
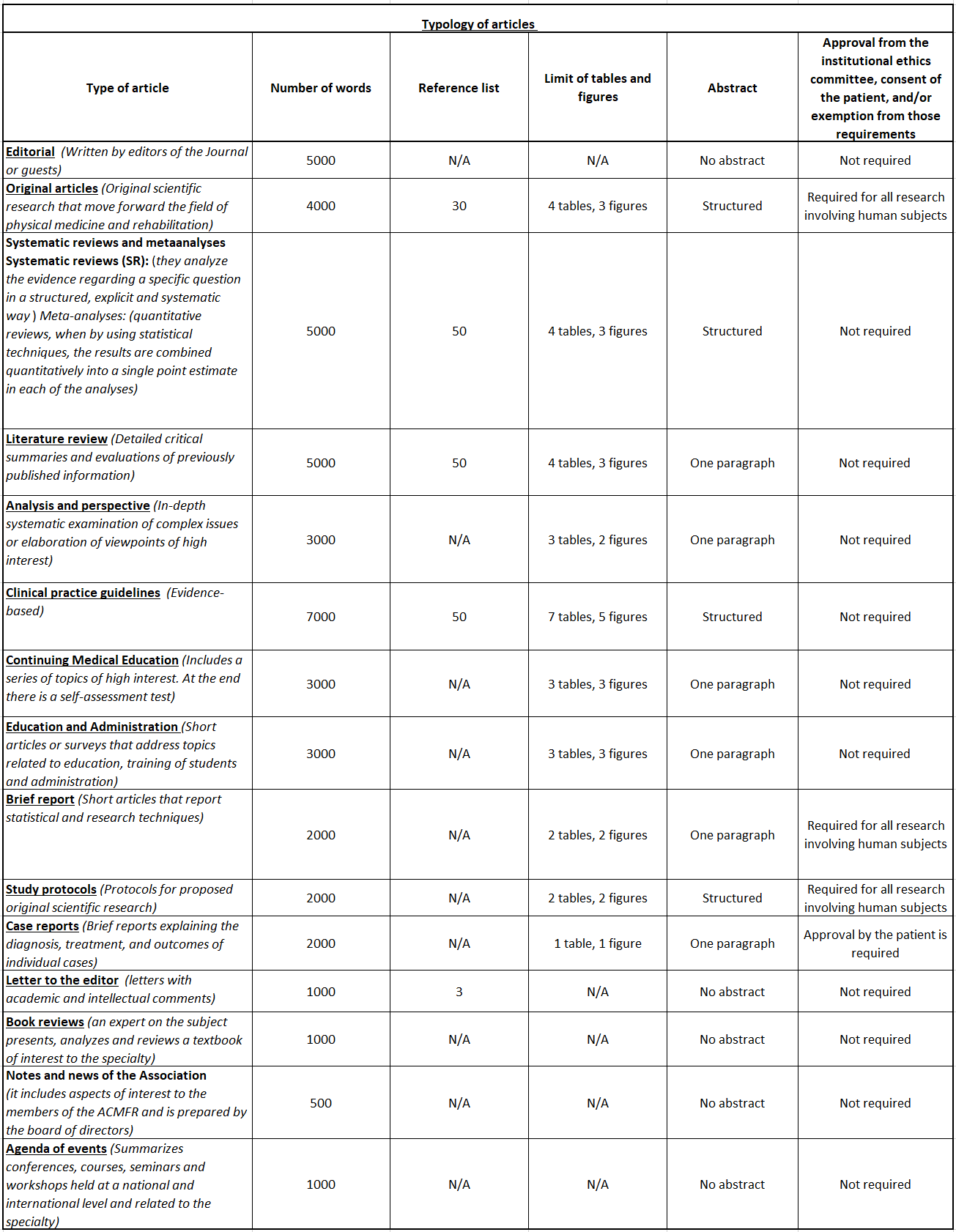
The length of the manuscripts varies according to the type of article: 4,000 words for original research articles; 5,000 words for systematic reviews, narrative reviews, and clinical practice guidelines; 3,000 words for articles on education and administration, and analysis and perspective; 2,000 words for brief reports and clinical cases, and 1,000 words for letters to the editor. The above figures correspond to the length of the text and do not include the title, tables, notes, figures, or references.
Sections of the Journal
Below we present the different types of articles that are published in the Rev Col Med Fis Rehab, which will be subject to the participation of the collaborators. Likewise, a table that summarizes the main characteristics of each of these types of articles is included.
Editorial: it is written by the editor or a guest of the editor and deals with topics on guild, educational, and of practice of the specialty.
Original articles: original clinical or basic research papers are presented and include systematic reviews, meta-analyses and clinical practice guidelines that are a contribution to the medical science.
Systematic reviews and meta-analyses: Systematic Reviews (SR): Are those that summarize and analyze the evidence regarding a specific question in a structured, explicit and systematic way. Meta-analyses: these are quantitative reviews when, through the use of statistical techniques, the results are quantitatively combined into a single point estimate in each of the analyses.
Literature reviews: they must be written by recognized authorities in each field or by request of the editor and should describe frequent and important topics in medical practice. In addition, they must have a minimum of 50 references.
Analyses and perspective: they correspond to a systematic in-depth examination of complex issues or to the elaboration of points of view of great interest.
Clinical Practice Guidelines: They must be based on evidence.
Continuing medical education articles: they include a series of topics of high interest for the specialty that are defined by the editorial committee. The invitation to participate in this section is made by the editor and a self-assessment test is included at the end of each article.
Education and administration: Short articles or surveys that address topics related to education, student training and administration.
Brief reports: these are short communications about research, technology or pedagogy.
Study Protocols: Protocols for proposed original scientific research.
Case Reports: they consist in the description or unique or illustrative cases of rare or interesting pathologies.
Letters to the editor: they include comments, suggestions and criticisms regarding the articles in the Journal or important topics of the practice of the specialty. A space to reply will be given to the author of the corresponding article.
Book reviews: the selection of books and reviewers for this section corresponds solely to the editor of the Journal.
Notes and news of the association: they include aspects of interest for the members of the ACMFR and are prepared by the board of directors.
Agenda of events: summarizes the congresses, courses, seminars and workshops held at a national and international level and related to the specialty.
N/A: not applicable.
Abbreviations and symbols
The abbreviations must be standardized and the first time they are included they must be preceded by the full term, except if it is a common unit of measurement; however, these should not be included in the title or in the abstract.
The medicines must be mentioned with their generic name.
Measurement units
The measurements of length, height, weight and volume must go according to the decimal metric system; those of blood pressure, in millimeters of mercury; those of temperature, in degrees centigrade, and the biochemical studies, in terms of the international system of units (SI). Decimals must include two digits and be separated by a comma.
Tables and figures.
As already mentioned, the tables and figures should be included in the text immediately after they are named in the text. In addition, they must have the following specific characteristics:
Tables: They must have a title and Arabic numerals according to the order of appearance and citation in the text. The title is placed at the top and the notes at the bottom. In the body of the table, the rows and columns that show a hierarchy must be clearly identified. The symbols for the units should appear in the column headings. The tables must have an initial horizontal line, then another below the items and a horizontal line at the end. They should not have vertical lines.
Figures: The photographs, graphs, drawings and shames are called figures; these, as well as the tables, are numbered with Arabic numbers according to the order in which they appear. In the case of photographs, they should be in color, with a minimum size of 300 dpi and in JPG or TIFF format, in addition, the persons must not be identifiable; otherwise they must be accompanied by a letter from the patient authorizing their publication. Statistical graphs and other illustrations should be professionally drawn in color. The title and legend should go at the bottom.
A maximum of 4 tables and 3 figures are accepted. In case that it is required to add more, an email must be sent to the editor justifying why the established number is exceeded.
All photographs, illustrations or any other previously published material must include the authorization of the original authors for their reproduction.
Structure
Original research articles, Review articles and meta-analysis should be structured as follows:
Title. It must contain less than 20 words, be easy to read and not include institutions.
Abstract. In Spanish and English (the same content in both languages). It should be structured (introduction, objectives, materials and methods, results and conclusion) and should not exceed 250 words or include abbreviations, acronyms or bibliographic references.
Keywords: 5 keywords in Spanish and 5 in English should be selected, which must be available in the DeCS (Health Sciences Descriptors) and MeSH (Medical Subject Headings) descriptors, respectively.
Introduction. It must be short; report on the state of the art of the subject of study; define the problem and the justification for the study, and end with the presentation of the purpose or objective of the study. It does not include tables or figures, but it includes the references necessary to contextualize the subject.
Methods. This section must clearly explain the design of the study (sample, calculation of the sample size, type of sampling and inclusion and exclusion criteria; the study population and the form of data collection (instruments, place, time and persons in charge); the appliances and devices used (manufacturer’s name and address in parenthesis), the procedures, the interventions and the observations carried out must also be identified in such a way that other researchers can reproduce the same conditions. In the event that the article is the product of a qualitative research, the categories of analysis and the theoretical reference used in its analysis must be registered. The statistical analysis, according to the objectives and the hypotheses raised, must contain the data that allow the verification of the results; in addition, the software used must be indicated.
The ethical aspects of the research must be described in the last paragraph of methods, in which it must be made clear that the guidelines for research on human beings or animals were followed (Declaration of Helsinki and ethical regulations of Colombia, Resolution 8430 of 1993 of the Ministry of Health). Likewise, if it is the case, it must be indicated that the informed consent from the participants to receive the treatment or to participate in the described research was obtained, as well as the approval by the ethics committees of the respective institutions, noting the number of minutes and date of approval. In the event that the patient can be recognized or identified through the images or data of the article, the author will declare that he/she has the informed consent for the publication of his/her data/images. The authors must declare that their publication does not contain personal information that allows to identify the patients. If experimentation on animals is reported, the agency or committee that authorized such experimentation must be mentioned.
Results. They must be presented in a logical sequence, with tables and illustrations accompanied by an explanation with their respective analysis. Not all the data of the tables and illustrations should be repeated in the text, and subheadings may be used to separate the findings. In this section, only what was found should be indicated, but without making an interpretation of the results or discussing their implications.
Discussion. In this section, the authors should explain what the implications for practice are, emphasis should be placed on the new and important aspects of the study, contrasting the results with the pertinent information available in the updated literature and relating them to the conclusions reached with the proposed objectives. The limitations and strengths of the study and the implications for practice and research should be included.
Conclusions. They must be directly related to the stated objective and supported by the results or findings of the study. It should be avoided to repeat the results. If it is pertinent in the study.
Acknowledgments and/or gratitude. The list of all those people who collaborated in the preparation of the article, but who do not meet the criteria of authorship, must be included.
Research funding. The author(s) must describe how the research was financed: write the institution and the number of the contract (if there is one).
Conflict of interest. The authors must explicitly declare whether or not they have potential conflicts of interest. This section is independent from the page of notification of conflict of interest that follows the title page, in which the authors provide additional details.
Citations and bibliographic references. Authors should ensure that the citations are no more than 10 years old and that at least 50% are less than 5 years old. The references, which must be numbered manually according to the order in which they appear in the text and must be identified with superscript Arabic numerals, must adhere to the updated Vancouver style standards, which can be consulted on the following pages: https://www.nlm.nih.gov/bsd/uniform_requirements.html
https://www.ncbi.nlm.nih.gov/books/NBK7256/
The names of the journals should be abbreviated according to the format of the Index Medicus.
Structure of the case reports
The case report articles must adhere to the CARE methodology https://www.clinicalcasereporting.com/es/empezar/reglas-generales
Title. It must contain less than 20 words, be easy to read and not include institutions.
Abstract. In Spanish and English (the same content in both languages). It must be structured: introduction, symptoms and important clinical findings of the patient, main diagnoses, presentation of the case and conclusion.
Signed copyright and authorship consent form. The authors must give their consent by signing this document.
https://www.clinicalcasereporting.com/es/empezar/reglas-generales. (Patient consent declaration form)
Examples of references
Journal article
Almeida-Fardin PB, De fúcio-Lizardo JH, da Silva-Baptista J. Study of the anterolateral ligament of the knee in formalin-embedded cadavers Acta Ortop Bras. 2017;25(2):89-92. Available from: https://doi.org/10.1590/1413-785220172502162204.
Zhang H, Qiu M, Xu Z, Wang W, Chen S, Zhang J, et al. The prevalence and morphological characteristics of the knee anterolateral ligament in a Chinese population. J Anat. 2018;233(2):213-21. Available from: https://doi.org/10.1111/joa.12826.
Book
Bozeman B, Boardman C. Research collaboration and team science: A state-of-the-art review and agenda. London: Springer; 2014.
Brown J. Nutrición en las diferentes etapas de vida. 2nd ed. Nueva York: Mc Graw Hill; 2006 [cited July 21, 2021]. Available from: https://www.academia.edu/42069760/Nutricion_en_la_Diferentes_Etapas_de_la_Vida_Brown.
Acts and resolutions
Colombia. Ministerio de Salud Pública. Decreto 786 de 1990 (abril 16): Por el cual se reglamenta parcialmente el título IX de la Ley 09 de 1979, en cuanto a la práctica de autopsias clínicas y médico-legales, así como viscerotomías y se dictan otras disposiciones. Bogotá D.C.: Diario Oficial 39300; 1990 [cited January 22, 2015]. Available from: https://www.icbf.gov.co/cargues/avance/docs/decreto_0786_1990.htm.
Colombia. Ministerio de Salud. Resolución 8430 de 1993 (octubre 4): Por la cual se establecen las normas científicas, técnicas y administrativas para la investigación en salud. Bogotá D.C.; 1993 [cited December 9, 2017]. Available from: https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/DE/DIJ/RESOLUCION-8430-DE-1993.PDF.
Thesis
Buitrago M. Discapacidades Peregrinas: Construcciones Sociales de la Discapacidad en Colombia: Aportes para la Salud Publica desde una mirada crítica [tesis]. Bogotá D.C.: Universidad Nacional de Colombia; 2013
Original articles
Original articles are those that present original clinical or basic research works that are a contribution to medical science, their length should not exceed 15 double-spaced pages.
Privacy Statement
The names and email addresses provided to the Journal will be used exclusively for the stated purposes and will not be made available to any other person or institution.
Any form of reproduction, distribution, public communication or transformation of this work can only be done with the prior authorization of the Colombian Association of Physical Medicine and Rehabilitation.

